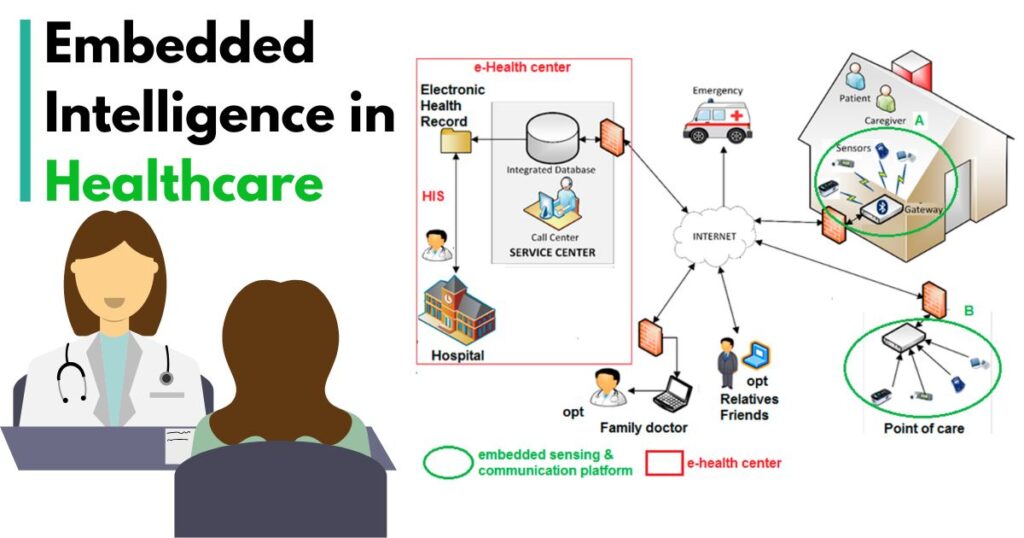
The medical device industry is undergoing a digital transformation powered by intelligent, embedded technologies. According to Fortune Business Insights, the global medical devices market was valued at USD 536.12 billion in 2023 and is projected to reach USD 799.67 billion by 2032, growing at a CAGR of 4.5%. Furthermore, MarketsandMarkets reports that software accounts for nearly 30% of the development costs of complex medical devices, showing the centrality of embedded systems in the modern healthcare ecosystem.
This growing reliance on technology means Embedded Software Development Services are no longer optional—they are integral to designing safe, compliant, and innovative medical solutions. From pacemakers to ventilators, embedded software ensures that these life-saving devices work accurately, efficiently, and securely.
What is Embedded Software in Medical Devices?
Embedded software refers to computer programs that are specifically written to operate hardware. In the medical context, these programs are embedded into diagnostic, therapeutic, and monitoring equipment. Unlike general-purpose software, embedded systems are often real-time, safety-critical, and designed for specialized tasks.
Examples of medical devices using embedded software:
- Implantable devices: Pacemakers, defibrillators
- Diagnostic tools: MRI scanners, ultrasound machines
- Therapeutic equipment: Infusion pumps, ventilators
- Monitoring systems: Glucose monitors, wearable ECGs
Key Roles of Embedded Software in Medical Device Development
1. Device Functionality and Precision
Embedded software controls how medical devices interpret and respond to inputs. It ensures precise operation and adherence to clinical requirements.
- Example: In insulin pumps, embedded software calculates and administers doses based on real-time glucose readings.
- Benefit: Minimizes human error and enhances patient safety.
2. Real-Time Data Processing
Many medical devices, especially in critical care, require immediate response to changing conditions. Embedded software enables real-time decision-making.
- Use Case: Heart monitors continuously analyze ECG signals and alert clinicians to arrhythmias instantly.
- Advantage: Timely interventions improve survival rates in emergency scenarios.
3. User Interface and Usability
Medical staff and patients interact with devices via software-driven interfaces. The embedded code powers these user experiences.
- Touchscreens, alerts, and control buttons all rely on responsive and intuitive embedded systems.
- Result: Streamlined workflows for healthcare providers.
Compliance and Regulatory Requirements
Medical devices must comply with strict regulatory standards globally. The embedded software plays a critical role in achieving these standards.
Major regulatory frameworks:
|
Region |
Regulatory Body |
Key Standard |
|
USA |
FDA |
21 CFR Part 820, IEC 62304 |
|
EU |
EMA |
MDR, ISO 13485 |
|
Global |
WHO |
ISO 14971, IEC 60601 |
Why compliance matters:
- Ensures patient safety
- Guarantees data integrity
- Reduces liability risks for manufacturers
How Embedded Software Development Services help:
- Provide documentation and version control needed for audits
- Integrate cybersecurity and safety features from the design stage
- Follow Software Development Life Cycle (SDLC) best practices mandated by standards
Integration with IoT and Wearable Health Devices
The rise of IoT in healthcare has dramatically expanded the scope of embedded software. Devices now collect and transmit data to cloud platforms and mobile apps.
Examples:
- Smart inhalers that track medication usage and sync data to an app
- Wearable ECG devices that stream data to clinicians in real-time
Key Embedded Software Capabilities:
- Wireless communication protocols (Bluetooth, Wi-Fi, Zigbee)
- Data encryption and secure transfer mechanisms
- Cloud integration APIs
Embedded Software and Cybersecurity
Medical devices are increasingly connected to hospital networks and the internet. This increases their attack surface.
Threats:
- Unauthorized access to device controls
- Theft of personal health data
- Device malfunction due to malware
Embedded software defenses include:
- Secure bootloaders
- End-to-end encryption
- Access control layers
- Regular OTA (Over-the-Air) software updates
Real-World Case:
In 2017, FDA recalled nearly 500,000 pacemakers due to vulnerabilities in wireless firmware. Updated embedded software mitigated the threat via patches.
Real-World Use Cases: Embedded Systems in Action
1. Philips Lumify Portable Ultrasound
- Uses embedded software to control image rendering and diagnostics.
- Offers smartphone integration via USB and Android apps.
2. Medtronic MiniMed Insulin Pump
- Embedded algorithms calculate dosage based on real-time blood glucose.
- Includes an automated feedback loop with continuous glucose monitors.
3. GE Healthcare CT Scanners
- Embedded control software enables real-time scanning, 3D image generation, and data encryption for HIPAA compliance.
Challenges in Developing Embedded Software for Medical Devices
Despite its advantages, building embedded software for medical applications poses unique challenges.
Key Challenges:
- Stringent validation and testing cycles
- Hardware-software compatibility issues
- Requirement for long-term support and maintenance
- Regulatory hurdles in multiple jurisdictions
These challenges highlight the need for specialized Embedded Software Development Services that have both technical expertise and domain knowledge.
Table: Comparison of Embedded Software in Consumer vs Medical Devices
|
Feature |
Consumer Devices |
Medical Devices |
|
Safety Requirements |
Low |
Extremely High |
|
Regulatory Oversight |
Minimal |
Extensive (FDA, IEC, ISO) |
|
Testing & Validation |
Basic QA |
Formal V&V and SDLC |
|
Software Update Cycles |
Frequent |
Rare, controlled releases |
|
Real-time Response Need |
Optional |
Often mandatory |
The Future of Embedded Software in Healthcare
As AI and machine learning continue to integrate with embedded systems, the potential applications in medicine are growing rapidly.
Emerging Trends:
- AI-powered diagnostic devices
- Autonomous robotic surgeries
- Smart prosthetics with embedded feedback loops
- Voice-enabled healthcare devices for elderly patients
The demand for robust, secure, and scalable Embedded Software Development Services will only increase as medical technology becomes more intelligent and interconnected.
Conclusion
Embedded software lies at the heart of today’s medical innovation. It powers everything from diagnostics and monitoring to therapy and surgical precision. Given the high stakes involved in patient care, investing in professional Embedded Software Development Services is crucial for creating reliable, safe, and compliant medical devices.
Companies that understand the regulatory landscape, can build secure real-time systems, and provide end-to-end embedded development support will lead the future of digital healthcare.
FAQs
Q1. Why is embedded software preferred in medical device design?
It offers precise control, real-time processing, and integrates well with sensors and actuators for life-saving decisions.
Q2. What standards must embedded software in medical devices comply with?
Common standards include IEC 62304, ISO 14971, and FDA’s 21 CFR Part 820.
Q3. Can medical devices receive software updates after deployment?
Yes, through secure OTA (Over-the-Air) updates with strict validation protocols.
Q4. What languages are commonly used in medical embedded software?
C, C++, and Assembly are popular, along with real-time OS platforms like FreeRTOS or VxWorks.
Q5. How do Embedded Software Development Services contribute to device security?
They implement encryption, access control, and secure data handling protocols from the development stage.
- Why Embedded Software is Essential for Medical Device Development | Embedded Software Development Services
- Discover why embedded software is critical for medical device development. Learn how expert Embedded Software Development Services ensure precision, compliance, and patient safety.
- embedded software in medical devices, medical device software development, embedded systems in healthcare, embedded software development services, FDA compliant software, medical IoT devices, real-time medical systems, medical device cybersecurity, embedded software testing, IEC 62304 compliant software
Related posts:
 Contrast Mapping for Dark Mode Accessibility in Taxi Booking Apps
Contrast Mapping for Dark Mode Accessibility in Taxi Booking Apps
 Boost Your Business with These App Development Companies in Kuwait
Boost Your Business with These App Development Companies in Kuwait
 How DevOps as a Service Accelerates Cloud Transformation for Modern Enterprises
How DevOps as a Service Accelerates Cloud Transformation for Modern Enterprises
 Retained vs. Contingent Search: Understanding the Value Dynamics Search Partners Brings to Each Model
Retained vs. Contingent Search: Understanding the Value Dynamics Search Partners Brings to Each Model
 Integrating MLM Software with E-Commerce Platforms: A Step-by-Step Guide
Integrating MLM Software with E-Commerce Platforms: A Step-by-Step Guide
 The Wimbo Revolution: Transforming Real-World Friendships in 2025
The Wimbo Revolution: Transforming Real-World Friendships in 2025
 How AI Call Assistants Are Helping Small Businesses Capture Every Opportunity
How AI Call Assistants Are Helping Small Businesses Capture Every Opportunity
 How Hotel Parking Management Software Transforms Guest Experience and Operational Efficiency
How Hotel Parking Management Software Transforms Guest Experience and Operational Efficiency