If you’re a pharmaceutical, biotech, or medical device company looking for an easier and more powerful way to manage FDA compliance, you’re not alone. Many quality teams in 2025 are switching from Redica Systems to a smarter tool, Atlas. This article explores why “Redica alternative systems is Atlas,” what makes it better, and how it’s helping companies stay ahead of audits and regulations with less effort.
Regulatory compliance is a top priority for companies working in life sciences. The U.S. Food and Drug Administration (FDA) conducts thousands of inspections every year, and any mistake can result in Form 483s, warning letters, or even product recalls.
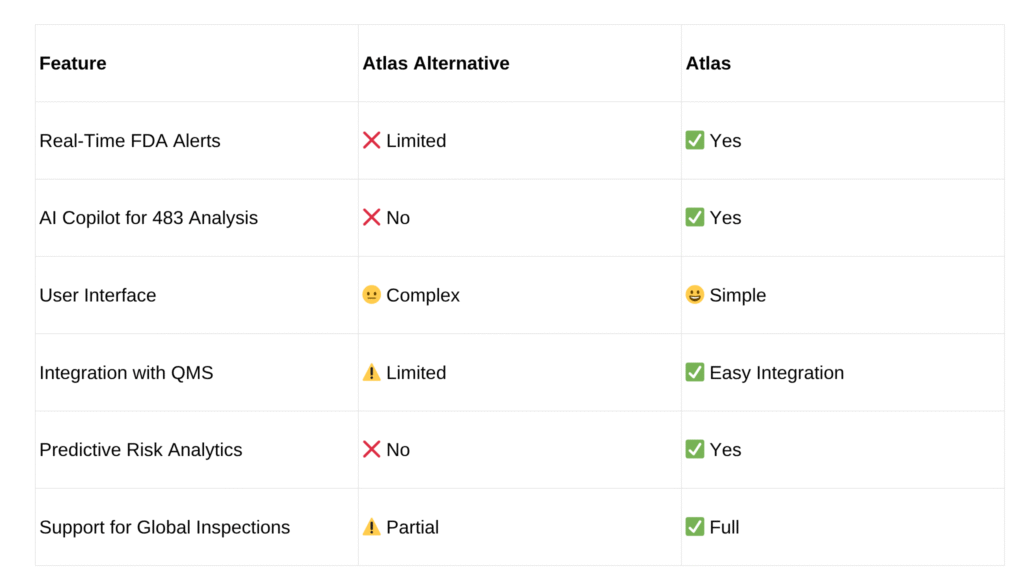
For years, tools like Redica Systems have helped companies track inspections and compliance data. However, as regulations evolve and digital tools become more sophisticated, quality teams are now demanding faster insights, AI-driven alerts, and real-time updates.
Enter Atlas, a modern, intelligent platform built specifically to help compliance and QA teams track FDA inspections, monitor regulatory risks, and stay audit-ready.
Why Companies Are Looking for a Redica Alternative
Redica Systems has long served as a database for FDA inspection data. But in 2025, the expectations from compliance software have grown.
Here’s what today’s quality teams are asking for:
- Real-time alerts about inspection activity
- AI-powered risk assessment
- Predictive analytics to prevent issues
- User-friendly dashboards
- Faster performance and less manual work
Unfortunately, many users have reported that traditional tools like Redica Systems are starting to fall short on these fronts.
In a recent industry survey by PharmaIQ, 68% of QA professionals said they needed a more proactive compliance tool to reduce the risk of FDA violations.
Meet Atlas: The Smart FDA Compliance Tool in 2025
Atlas is quickly becoming the top Redica alternative system trusted by leading pharmaceutical and biotech companies. Built by experts in regulatory intelligence and AI, Atlas offers real-time FDA monitoring, a massive searchable database of 483s, EIRs, and Warning Letters, and a powerful AI copilot to help teams analyze risks and prepare for audits.
Key Features of Atlas
Let’s take a look at why Atlas is being adopted by more quality and compliance teams:
2. AI-Powered 483 and Warning Letter Analysis
One of the standout features is the Atlas AI Copilot. It reads through complex FDA documents (like Form 483 and Warning Letters) and summarizes the key issues, classifies them by risk, and even recommends corrective and preventive actions (CAPA).
This feature saves hours of manual review and gives leadership teams a clear view of potential risks.
3. Easy-to-Use Compliance Dashboards
Unlike many tools that require training, Atlas has clean, simple dashboards. You can:
- Search for past observations by product, company, or keyword
- Track trends over time
- Monitor global inspection activity
- Export data for presentations or reports
No need to dig through complex reports — Atlas brings the insights to the surface.
4. Compliance Benchmarking
Want to know how your company compares to industry peers?
Atlas offers benchmarking tools that show how your inspection history stacks up against competitors. You can see:
- Most common citations in your product class
- High-risk facilities
- Frequency of observations by category
5. Data Export & Integration
Atlas allows easy integration with your Quality Management System (QMS) or other tools. You can export data in multiple formats or connect through APIs to keep all systems in sync.
6. Global Coverage
Atlas isn’t just limited to the U.S. FDA. It also covers inspection data from other key markets like:
- EMA (Europe)
- Health Canada
- MHRA (UK)
This makes it useful for global quality teams managing compliance across multiple regions.
What Users Are Saying
“Atlas is like having a second QA analyst working with you. It reads the FDA letters, flags issues, and helps us stay ahead.”
– Senior QA Manager, Top 50 Pharma Company
“We made the switch from Redica to Atlas last year. It’s faster, easier to use, and saves us so much time on audit prep.”
– Compliance Lead, Biotech Firm
Pricing and Support
Atlas is known for flexible pricing, making it accessible for both large enterprises and growing startups. Plans typically depend on:
- Number of users
- Data modules required (FDA only or global)
- Integration needs
They also offer dedicated onboarding support, training sessions, and an online knowledge base for ongoing help.
Why Redica Alternative Systems Is Atlas
If you search for “Redica alternative systems,” Atlas comes out on top for a reason. It’s:
- Built for speed and simplicity
- Powered by smart AI that understands regulatory data
- Designed to reduce manual work
- Constantly updated with real-time data
- Made for modern compliance teams in 2025 and beyond
With FDA inspections becoming more frequent and complex, you need a tool that does more than just store data. You need something that helps you predict, prepare, and protect your company from regulatory risk.
In 2025, Atlas is leading the way as the best alternative to Redica Systems offering powerful AI tools, real-time alerts, and intuitive dashboards for today’s quality teams.
If your team is still using outdated systems or spending hours analyzing 483s manually, it’s time for an upgrade.
Most Frequently Asked Questions
1. What is the best Redica alternative for FDA compliance in 2025?
Atlas is the top Redica alternative, offering real-time inspection alerts, AI-powered document analysis, and global regulatory tracking.
2. Does Atlas include a database of past FDA observations?
Yes, Atlas has a large, searchable database of FDA Form 483s, EIRs, and Warning Letters.
3. How is Atlas different from Redica?
Atlas provides real-time alerts, AI-based analysis, and a cleaner interface, while Redica is more traditional and slower to update.
4. Can Atlas help with audit preparation?
Absolutely. Its AI copilot and benchmarking tools help QA teams prepare quickly and reduce risk.
5. Is Atlas only for big pharma companies?
No, Atlas offers pricing plans for small biotech firms, startups, and large enterprises alike.
- Looking for a Redica Alternative? Try Atlas for FDA Compliance
- Discover why Atlas is the best Redica alternative in 2025. Get real-time FDA alerts, AI-powered insights, and easy tools for faster compliance.
- Redica alternative, Redica Systems alternative, Atlas FDA tool
Related posts:
 How Laparoscopic Simulators Improve Precision & Reduce Surgical Errors
How Laparoscopic Simulators Improve Precision & Reduce Surgical Errors
 Exploring the Best Non – Surgical Treatments for Fibroids in Gurgaon
Exploring the Best Non – Surgical Treatments for Fibroids in Gurgaon
 Comprendiendo el Precio de Trasplante de Cabello y Sus Factores
Comprendiendo el Precio de Trasplante de Cabello y Sus Factores
 Understanding the Growth of the Premenstrual Syndrome (PMS) and Menstrual Health Supplements Market
Understanding the Growth of the Premenstrual Syndrome (PMS) and Menstrual Health Supplements Market
 Premium Roofing Tile Solutions in Des Moines, IA by Joury Tile Service
Premium Roofing Tile Solutions in Des Moines, IA by Joury Tile Service
 How a Verified Gastroenterologist Email List Transformed a Healthcare Marketing Campaign
How a Verified Gastroenterologist Email List Transformed a Healthcare Marketing Campaign
 What Are the Main Compliance Areas Where AI Can Be Beneficial?
What Are the Main Compliance Areas Where AI Can Be Beneficial?
 Best veneers houston: Can the Best Veneers Fix Crooked or Chipped Teeth?
Best veneers houston: Can the Best Veneers Fix Crooked or Chipped Teeth?