Introduction to Non-coding RNA Epigenetics
Non-coding RNAs (ncRNAs) are RNA molecules that do not encode proteins but regulate gene expression through various mechanisms. In recent years, ncRNAs have emerged as critical regulators of epigenetic processes, drawing increasing attention for their roles in both health and disease. This review explores the epigenetic functions of ncRNAs and the role of bioinformatics in elucidating their complex regulatory networks.
Role of ncRNAs in Epigenetic Regulation
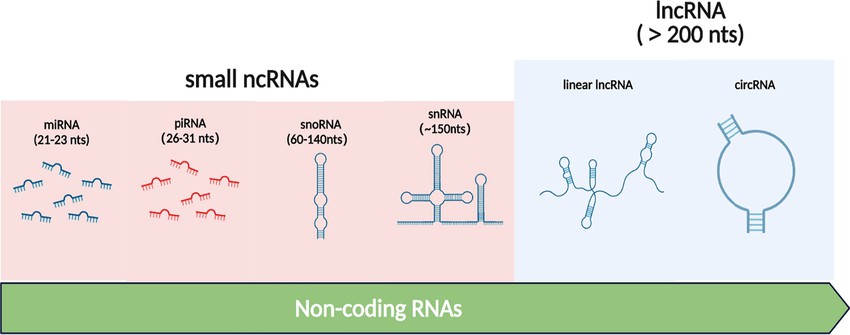
Non-coding RNAs encompass a diverse group of molecules, including microRNAs (miRNAs), small interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs), and long non-coding RNAs (lncRNAs). These ncRNAs modulate gene expression at transcriptional and post-transcriptional levels, influencing chromatin dynamics, DNA methylation, histone modifications, and RNA stability. The primary mechanisms through which ncRNAs exert epigenetic control are summarized below:
miRNAs: Small (~22 nucleotides) regulatory RNAs that bind to complementary sequences on target mRNAs, leading to their degradation or translational repression. miRNAs play significant roles in gene silencing and are implicated in various diseases, including cancer.
siRNAs: Double-stranded RNA molecules that guide the RNA-induced silencing complex (RISC) to complementary mRNA targets, facilitating mRNA cleavage and gene silencing. siRNAs are primarily involved in antiviral defense and transposon silencing.
piRNAs: A distinct class of small RNAs that interact with PIWI proteins, primarily functioning in germline cells to silence transposable elements and maintain genome integrity.
lncRNAs: RNA molecules longer than 200 nucleotides that regulate gene expression through diverse mechanisms, including chromatin remodeling, transcriptional interference, and acting as molecular scaffolds. lncRNAs are involved in a wide range of cellular processes, from X-chromosome inactivation to the regulation of developmental pathways.

Figure 1. Types of Non-coding RNAs (Jie-yu Sun et al,. 2021)
Mechanisms of ncRNA-Mediated Gene Regulation
ncRNA molecules regulate gene expression through interactions with DNA, RNA, or proteins, utilizing various mechanisms to control cellular growth, differentiation, and function. Recent studies have demonstrated that ncRNAs also modulate epigenetic marks, such as DNA methylation and histone modifications, thereby influencing gene expression.
ncRNAs function through diverse pathways, impacting gene expression at multiple levels:
Interaction with DNA: ncRNAs can guide chromatin-modifying complexes to specific genomic loci, influencing chromatin state and accessibility. For instance, lncRNAs can recruit DNA methyltransferases, leading to targeted DNA methylation, which suppresses gene transcription.
RNA Interference: Small ncRNAs, such as miRNAs and siRNAs, interact with complementary RNA sequences to mediate post-transcriptional gene silencing. These molecules are integral to the RNA-induced silencing complex (RISC), where they guide the complex to target mRNAs, resulting in degradation or translational repression.
Histone Modification: ncRNAs influence histone modifications, such as acetylation, methylation, and phosphorylation, which are key determinants of chromatin structure and gene expression. For example, certain lncRNAs serve as scaffolds for chromatin-modifying enzymes, directing them to specific histone residues to alter chromatin conformation and regulate transcriptional activity.
Modulation of Epigenetic Landscapes: Emerging evidence suggests that ncRNAs are critical in shaping the epigenetic landscape by directly influencing DNA methylation patterns and histone modifications. This regulation can be gene-specific or genome-wide, contributing to the dynamic control of gene expression in response to cellular signals and environmental changes.

Figure 2. LncRNA-mediated gene regulation mechanisms. (Subhasree Kumar et al,. 2022)
ncRNAs Epigenetic Research Techniques
Epigenetic research on ncRNAs represents a rapidly evolving field with substantial potential for elucidating gene regulation and disease pathogenesis. The study of ncRNA-mediated epigenetic regulation is facilitated by various advanced techniques that allow for the exploration of ncRNA expression patterns, interactions with epigenetic marks, and functional roles in different cellular contexts. The following methodologies are commonly employed in the study of ncRNA epigenetics:
1. RNA Sequencing (RNA-seq)
RNA sequencing is a powerful technique employed to determine differential expression patterns of ncRNAs. This method enables the identification of ncRNAs that exhibit variable expression across different cells or tissues, providing insights into the roles of ncRNAs in epigenetic regulation. By analyzing the expression profiles of ncRNAs, researchers can elucidate their contributions to gene expression modulation in health and disease.
2. Chromatin Immunoprecipitation Sequencing (ChIP-seq)
ChIP-seq is a widely utilized technique for identifying the interactions between ncRNAs and epigenetic marks. This approach aids in determining how ncRNAs regulate specific gene expression by examining their interactions with chromatin-associated proteins and epigenetic modifications such as histone marks. ChIP-seq provides valuable data on the regulatory networks mediated by ncRNAs and their influence on chromatin dynamics.
3. Immunoprecipitation (IP) Techniques
Immunoprecipitation techniques, including RNA immunoprecipitation (RIP) and crosslinking immunoprecipitation (CLIP), are employed to identify interactions between ncRNAs and proteins. By isolating complexes of ncRNAs with specific proteins, these methods provide insights into the mechanistic roles of ncRNAs in modulating epigenetic marks. The identification of protein partners of ncRNAs enhances the understanding of their functional roles in gene regulation.
4. In Situ Hybridization (ISH)
In situ hybridization is a pivotal technique used to study the spatial expression patterns of ncRNAs within cells and tissues. This method enables the localization of ncRNAs at the subcellular level, revealing their distribution and providing clues about their functional roles in epigenetic regulation. The precise mapping of ncRNA expression helps elucidate their involvement in specific cellular processes.
5. CRISPR-Cas9 Technology
CRISPR-Cas9 genome editing technology is instrumental in studying the functional roles of ncRNAs in epigenetic regulation by enabling targeted knockout or overexpression of specific ncRNAs. By manipulating ncRNA expression, researchers can investigate the effects on gene expression and epigenetic marks, thereby uncovering the regulatory mechanisms governed by ncRNAs.
6. Single-Cell Sequencing
Single-cell sequencing techniques allow for the investigation of ncRNA expression at the single-cell level, revealing expression heterogeneity among different cell types. This approach provides a high-resolution view of ncRNA function in epigenetic regulation, highlighting cell-specific roles and regulatory patterns that are often obscured in bulk analysis. The ability to analyze individual cells enhances the understanding of ncRNA-mediated gene regulation in complex tissues.
Design of Epigenetic Studies on ncRNAs
The investigation of epigenetic mechanisms involving ncRNAs requires a systematic approach to address relevant biological questions, define the study population, collect and analyze data, validate findings, and explore potential clinical applications. The following steps outline a general framework commonly employed in the study of ncRNA-mediated epigenetic regulation:
1. Formulation of the Research Question
The initial step involves the identification of the specific research question. This may include investigating the role of ncRNAs in a particular disease or pathological condition, or identifying novel ncRNAs implicated in epigenetic regulation. Defining the research question provides a clear focus and guides the subsequent experimental design.
2. Definition of the Study Population
The definition of the study population is critical and should align with the research question. This may involve selecting patients with a specific disease, healthy controls, or other relevant groups. The choice of study population determines the scope of the investigation and influences data interpretation.
3. Collection and Analysis of Genomic Data
Genomic data are typically collected from public databases or generated using advanced techniques such as RNA-seq and ChIP-seq. These technologies facilitate the identification of differentially expressed ncRNAs and associated epigenetic marks. Bioinformatics tools are then employed to analyze the data, elucidating the relationships between ncRNAs and epigenetic modifications.
4. Validation of Study Findings
Upon identifying candidate ncRNAs and epigenetic marks, it is essential to validate the results using additional experimental approaches, such as quantitative polymerase chain reaction (qPCR) or in situ hybridization. These validation steps confirm the initial findings and ensure the reliability of the identified ncRNA-epigenetic interactions.
5. Functional Analysis
Once candidate ncRNAs have been validated, functional analysis is conducted to elucidate their roles in epigenetic regulation. This may involve downstream target analysis, such as knockdown or overexpression experiments, to assess the impact of ncRNAs on gene expression and epigenetic modifications. Functional assays provide insights into the mechanistic pathways through which ncRNAs influence cellular processes.
6. Clinical Applications
If the ncRNAs are found to play significant roles in epigenetic regulation, further research may explore their potential as biomarkers or therapeutic targets. Clinical applications could involve evaluating ncRNAs as diagnostic tools, prognostic indicators, or therapeutic modulators, thereby expanding their relevance in personalized medicine.
Current Landscape and Future Prospects
With the rapid advancements in epigenetics, the roles of ncRNAs in cellular biology have garnered significant attention. Accumulating evidence suggests that ncRNAs function through various mechanisms, including the regulation of gene expression, modulation of cellular differentiation, and participation in disease pathogenesis. Consequently, exploring the epigenetic landscapes of ncRNAs has emerged as a critical area of contemporary research.
The investigation of ncRNA epigenetics offers valuable insights for disease diagnosis and therapeutic interventions. For instance, several studies have demonstrated that ncRNAs play pivotal roles in the initiation and progression of tumors, highlighting the potential of ncRNA epigenetic landscapes as novel diagnostic and therapeutic targets in oncology. Furthermore, the study of ncRNA epigenetics can elucidate the mechanisms of cellular differentiation, providing a deeper understanding of ncRNAs in developmental biology, immune regulation, and growth processes.
As epigenetic technologies continue to evolve, the research on ncRNA epigenetic landscapes is poised for substantial growth. The advent of single-cell sequencing technologies enables high-resolution analysis of ncRNA expression and regulatory dynamics at the individual cell level. Additionally, emerging epigenetic techniques and analytical methods promise to enhance the precision and depth of ncRNA epigenetic landscape studies, offering new avenues for scientific exploration.
References
- Wei, J.-W., Huang, K., Yang, C., & Kang, C.-S. (2017). Non-coding RNAs as regulators in epigenetics (Review). Oncology Reports.
- Epigenetics of Non-coding RNA (ncRNA): An Overview of Bioinformatics
- Explore the epigenetic roles of ncRNAs and bioinformatics techniques in gene regulation. Discover advanced research methods and bioinformatics tools used to study ncRNA epigenetics.
- biotech,health,
Related posts:
 Best Topical Finasteride & Minoxidil Spray for Hair Regrowth
Best Topical Finasteride & Minoxidil Spray for Hair Regrowth
 What Are the Key Considerations Before Getting Filler Injections?
What Are the Key Considerations Before Getting Filler Injections?
 Dr. Kami Hoss Gives Out the Truth About Brushing & Flossing-Protecting Your Teeth
Dr. Kami Hoss Gives Out the Truth About Brushing & Flossing-Protecting Your Teeth
 Effective Weight Loss Clinic Killeen: Your Path to Lasting Results
Effective Weight Loss Clinic Killeen: Your Path to Lasting Results
 How to Prepare for Your Appointment with the Best Plastic Surgeon in dubai for Fillers
How to Prepare for Your Appointment with the Best Plastic Surgeon in dubai for Fillers
 Comment les analgésiques sur ordonnance se comparent-ils aux options en vente libre ?
Comment les analgésiques sur ordonnance se comparent-ils aux options en vente libre ?
 Prozenith: A Natural Weight Loss Supplement 70% OFF Discount
Prozenith: A Natural Weight Loss Supplement 70% OFF Discount
 Recovery and Aftercare Tips for Gynecomastia Patients in Dubai
Recovery and Aftercare Tips for Gynecomastia Patients in Dubai