Introduction
Central Precocious Puberty (CPP) is a condition where puberty begins significantly earlier than normal, typically before the age of 8 in girls and 9 in boys. It results from premature activation of the hypothalamic-pituitary-gonadal (HPG) axis and often requires timely medical intervention to prevent adverse psychosocial and physical outcomes. In North America, awareness and early diagnosis of CPP have improved, leading to greater demand for effective treatment options.
This article explores the North America CPP treatment market, analyzing its key drivers, medical advancements, patient demographics, market segmentation, and future prospects through 2033.
Market Overview
As of 2024, the North America Central Precocious Puberty (CPP) treatment market is valued at approximately USD 640 million. The market is expected to reach USD 1.1 billion by 2033, growing at a compound annual growth rate (CAGR) of 6.2%. The United States accounts for the majority share due to high healthcare expenditure, established diagnostic protocols, and availability of advanced therapeutic options.
Key Market Drivers
Rising Prevalence of CPP
Improved diagnostic techniques and increased pediatric healthcare visits have contributed to the rising detection of CPP cases, leading to higher treatment rates.
Increased Awareness Among Parents and Physicians
Educational initiatives and enhanced understanding of CPP symptoms are prompting earlier consultations and timely interventions.
Advancements in GnRH Analogue Therapy
GnRH (gonadotropin-releasing hormone) agonists are the standard treatment for CPP. Innovations in drug formulations, such as long-acting injectables and subcutaneous implants, are improving treatment outcomes and compliance.
Pediatric Endocrinology Specialization Growth
The expansion of pediatric endocrinology as a clinical specialty across North America ensures more accurate diagnosis and management of CPP.
Favorable Insurance Coverage
Health insurance policies in both the U.S. and Canada typically cover CPP treatments, making it more accessible for affected families.
Market Segmentation
By Drug Type:
GnRH Agonists:
Leuprolide Acetate
Triptorelin
Histrelin Acetate
By Route of Administration:
Intramuscular Injection
Subcutaneous Implant
Subcutaneous Injection
By End User:
Hospitals
Specialty Clinics
Pediatric Endocrinology Centers
Homecare Settings
By Country:
United States
Canada
Therapeutic Landscape
GnRH Agonists:
The most widely used treatment approach for CPP involves GnRH agonists, which act by suppressing pituitary hormone release, thereby halting premature sexual development.
Leuprolide Acetate (e.g., Lupron Depot-Ped): Administered monthly or quarterly, widely used in clinical settings.
Triptorelin: Offers long-acting formulations, reducing the frequency of injections.
Histrelin Implants (e.g., Supprelin LA): One-year subcutaneous implant, minimizing compliance challenges and clinic visits.
Regional Insights
United States:
The U.S. leads the regional market with high diagnosis rates, presence of top-tier pediatric hospitals, and strong pharmaceutical R&D. Initiatives by pediatric associations and wider health literacy contribute to the country’s dominance.
Canada:
Canada’s market is expanding with growing recognition of CPP as a manageable endocrine disorder. Public healthcare coverage and increasing access to pediatric endocrinologists are supporting market development.
Technological and Clinical Trends
Extended-Release Formulations
Long-acting drugs and implants reduce the burden of frequent injections, improve adherence, and enhance quality of life for children undergoing CPP treatment.
Genetic and Biomarker Research
Advancements in genetic profiling and biomarker identification are improving diagnosis accuracy and enabling more personalized treatment.
Telehealth and Remote Monitoring
The use of telemedicine for follow-ups and dose adjustments is making CPP management more convenient, especially in rural or underserved areas.
Combination Therapy Investigations
Some research is exploring adjunctive treatments to optimize hormone regulation and manage side effects, although GnRH agonists remain the gold standard.
Market Challenges
High Treatment Costs
Despite insurance coverage, the cost of long-term GnRH therapy can be a financial burden for families without comprehensive health plans.
Limited Access to Specialists
In rural areas, lack of pediatric endocrinologists may lead to delayed diagnosis or suboptimal treatment, especially in Canada.
Emotional and Psychological Impact
CPP can affect children’s mental health. There is a need for integrated care involving both endocrinologists and pediatric psychologists.
Lack of Awareness in Underserved Communities
CPP is still under-recognized in certain demographic groups, leading to delayed treatment initiation and poorer outcomes.
Competitive Landscape
Major players operating in the North America CPP treatment market include:
AbbVie Inc.
Ferring Pharmaceuticals
Pfizer Inc.
Sun Pharmaceutical Industries Ltd.
Tolmar Pharmaceuticals, Inc.
Ipsen
Endo International plc
Debiopharm Group
Arbor Pharmaceuticals
These companies focus on R&D for extended-release drug delivery systems, regulatory approvals, and physician outreach programs.
Future Outlook (2025–2033)
Increased adoption of long-acting implants and less invasive delivery systems
Broader use of AI and digital tools in CPP diagnostics and treatment planning
Expansion of CPP awareness campaigns targeting school health programs and community clinics
Enhanced focus on comprehensive care models that combine medical, psychological, and social support
Regulatory push for earlier screening in pediatric care settings
Conclusion
The North America Central Precocious Puberty (CPP) treatment market is poised for continued growth, driven by technological advancements, rising diagnosis rates, and improved healthcare access. While barriers such as cost and limited specialist availability persist, the overall trajectory remains positive. Ongoing innovation in treatment options and integrated care delivery will ensure that children with CPP receive timely, effective, and compassionate care. Stakeholders that prioritize education, accessibility, and long-term treatment outcomes will shape the future of CPP management across North America.
- North America Central Precocious Puberty (CPP) Treatment Market: Trends, Therapeutic Advances, and Growth Outlook to 2033
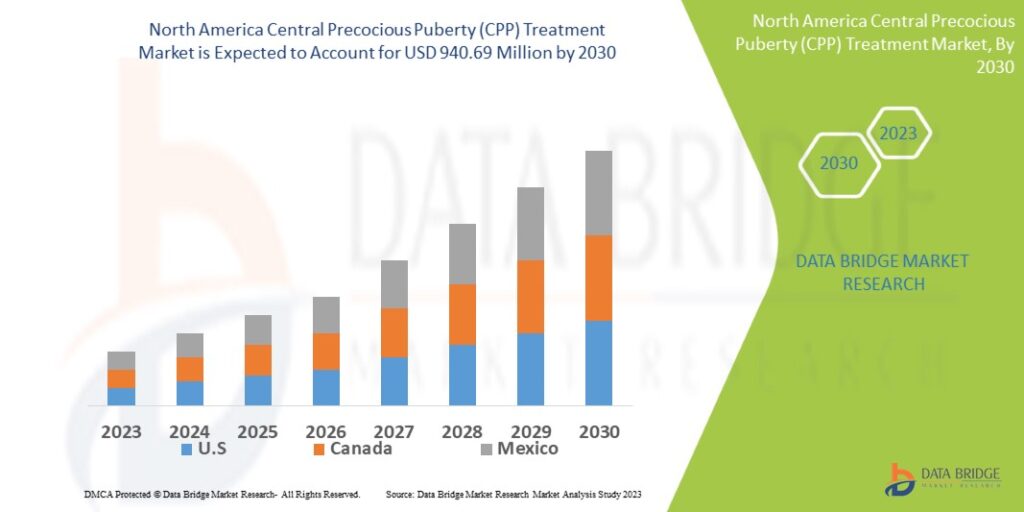
- Data Bridge Market Research analyses a growth rate in the central precocious puberty (CPP) treatment market in the forecast period 2023-2030. The expected CAGR of central precocious puberty (CPP) treatment market is tend to be around 6% in the mentioned forecast period. The market was valued at USD 590.2 million in 2022, and it would grow up to USD 940.69 million by 2030.
- North America Central Precocious Puberty (CPP) Treatment Market
Related posts:
 Nutrition and Wellness Programs in Assisted Living Communities in Oakville
Nutrition and Wellness Programs in Assisted Living Communities in Oakville
 How UAE Fitout Experts Are Elevating Luxury Brand Spaces in 2025
How UAE Fitout Experts Are Elevating Luxury Brand Spaces in 2025
 Sp5der Hoodie: Comfort, Fit, and Real-World Functionality Redefined
Sp5der Hoodie: Comfort, Fit, and Real-World Functionality Redefined
 Boca Raton Auto Insurance: Online Quotes from Leading Providers
Boca Raton Auto Insurance: Online Quotes from Leading Providers
 Learn Indian Stock Market Course at ICFM – The Trusted Institute for Career-Driven Financial Learning
Learn Indian Stock Market Course at ICFM – The Trusted Institute for Career-Driven Financial Learning
 How To Make Your Spray Tan Last Longer: 7 Pro Tips Honey Hue Tans
How To Make Your Spray Tan Last Longer: 7 Pro Tips Honey Hue Tans
 Choosing the Right Tank Size for Your Mosquito Misting System
Choosing the Right Tank Size for Your Mosquito Misting System
 Modern Flexible Office Pods for Stylish, Productive Workspaces
Modern Flexible Office Pods for Stylish, Productive Workspaces